
Cell Applications, Inc.
Interested in working with
Cell Applications, Inc.?
Overview
Global Leadership in Primary Cells
Excellence in primary cell isolation, optimized culture media, Induced Pluripotent Stem Cells (iPSC) & other stem cells, 3D cell models, cellular diseasse models, cell bioprinting and more. CAI's products and custom services support basic life science research and biopharmaceutical drug discovery. Our Center for Primary Cell Innovation pushes the edges of technology, with regular advancements developed in collaboration with academic & industry experts.
Standards & Methodologies
With a full cell culture toolbox, we hold deep experience isolating physiologically relevant primary cells directly from living tissue. Our Custom services include Primary Cell Isolation, where we also expand, freeze, document and test primary cells isolated from nearly any tissue or species. Choices include normal & diseased cells, unique donor profiles and different cell types from the same donor. Superior cell culture systems are optimized for each cell, in order to maintain optimal cell health and behavior. Primary cells are carefully removed and released from their physiological environment to establish a growing population in a controlled environment. Our 3D Cell Bioprinting approach takes advantage of our expansive library of primary cells and iPSC-derived lineages for engineering of tissues and organoids. Induced Pluripotent Stem Cell (iPSC) capabilities include cells, media and custom iPSC generation & differentiation.
Qualifications, Certifications, Accreditations & Licenses
cGMP (FDA 21 CFR Part 820): Current Good Manufacturing Practice for Medical Devices. Pertains to the manufacturing and packaging of human and animal cell culture media. ISO (9001:2008): Applies to the manufacture and supply of human and animal cells and related products for research use.
Lab Gallery



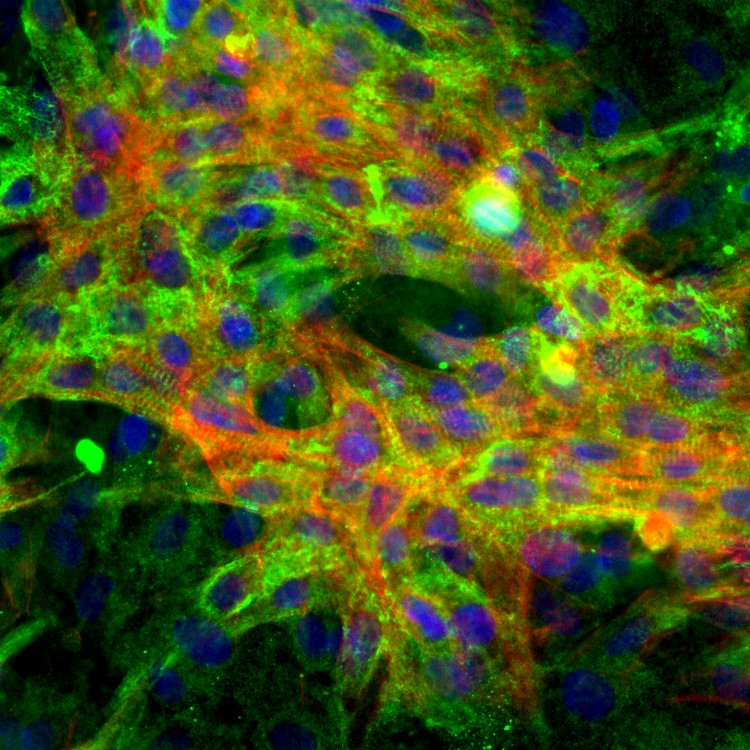

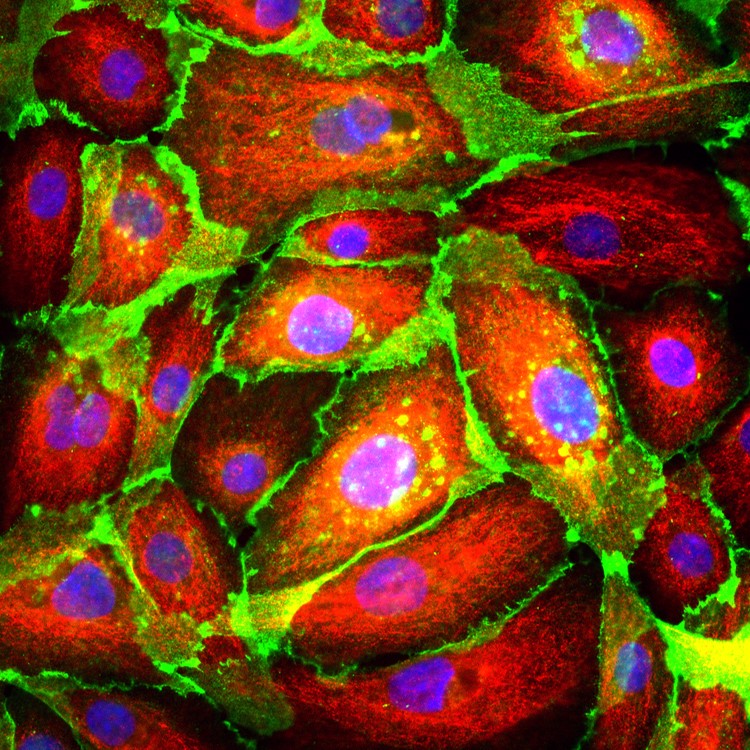
Industries Served
- Biologics
- Biotechnology
- Cell Lines and Cell Cultures
- Genomics and Genetics
- Healthcare and Medical
- In-Vitro Diagnostics
- Life Sciences
- Pharmaceutical
- Proteins and Peptides
- Veterinary Medicine
Testing Types
- Animal Science
- Biochemistry
- Biocompatibility
- Biology
- Biomaterials
- Biomechanical
- Biomedical
- Biomedical Engineering
- Cytology
- Cytopathology
- Dermatology
- Genomics
- Genotoxicity
- Immunoassays
- Immunofluorescence
- Immunohistochemistry
- Immunology
- In-Vitro
- Method Development
- Microscopy and Imaging
- Modeling
- Molecular Biology
- Pharmacology
- Physiology
- Preclinical
- Product Development
- Protein Characterization
- Tissue Engineering
- Toxicity
- Toxicology
- Virology / Virus
Publications
Induced Pluripotent Stem Cells & iPSC-Derived Lineages for Life Science R&D (Article)Induced Pluripotent Stem Cells & iPSC-Derived Lineages for Life Science R&D (White Paper)
Beating Heart "Organoid" engineered using 3D Bioprinting and iPSC-Derived Heart Cells, Nov 8, 2016 (Press Release)
Beating Heart "Organoid" engineered using 3D Bioprinting and iPSC-Derived Heart Cells, Nov 8, 2016 (Press Release)
CAI Indicates the Upcoming Launch of Human iPSC-Derived Cardiomyocytes (i-HCm), Dec 2, 2016 (Press Release)
Perineurial Fibroblasts and Schwann Cells Introduced at CAI, Oct 25, 2016 (Press Release)
CAI Introduces Human Trabecular Meshwork Cells, Sep 27, 2016 (Press Release)
Primary Cells Shed New Light on Vascular Physiology and Disease (Article)
Why Primary Cells? (Case Study)
Cell Applications, Inc. Introduces New Primary Cells (Press Release)
The Case for Primary Cells: (Article)
New scaffold-free 3-D bioprinting method available for first time in North America (Press Release)
Cell Applications and StemoniX Accelerate Life Science Research with Mass Production of Human Induced Pluripotent Stem Cells (Press Release)
DISCLAIMER: This Laboratory Profile was provided by the company above.
