.png)
Solvias USA, Inc.
Interested in working with
Solvias USA, Inc.?
Overview
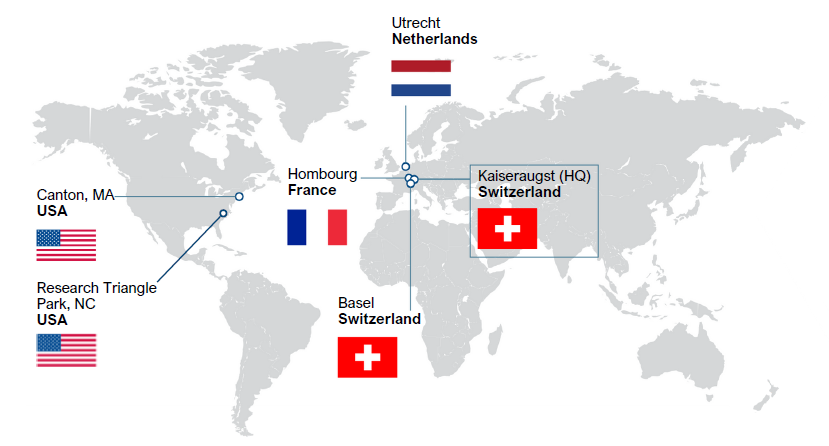
Solvias is a world leading CRO/CDMO headquartered in Switzerland with 6 sites across the U.S. & EU. Drawing on a proven 25-year track record of scientific excellence, we provide cGMP-compliant contract analytical services to help you bring safe and effective therapies to patients faster. Partner with us to gain access to a wide range of capabilities for characterization of biologics, ATMPs and small molecule drugs and method development, release testing and stability services. We also offer one of the largest ligand portfolios for catalytic transformations and a suite of related custom API synthesis and catalysis technology services.
Standards & Methodologies
USP, EP, JP, ISO
Qualifications, Certifications, Accreditations & Licenses
ISO9001, ISO17025, GMP, GLP, 21 CFR Part 11, DEA, FDA inspected
Lab Gallery






Industries Served
- Biopharmaceuticals
- Biotechnology
- Cell Lines and Cell Cultures
- Gene Therapy
- Laboratories or Research Facilities
- Laboratories or Research Facilities
- Life Sciences
- Medical Devices
- Packages and Packaging
- Pharmaceutical
- Pharmaceutical - Pediatric
- Pharmaceutical - Active Pharmaceutical Ingredients (API)
- Pharmaceutical - Compounding
- Pharmaceutical - Over the Counter (OTC)
- Pharmaceutical - Small Molecules
- Pharmaceutical - Sterile
- Pharmaceutical Excipients
- Pharmaceuticals - Large Molecules
- Veterinary Medicine
Testing Types
- Amino Acid Analysis
- Analytical Chemistry
- Antibody-Based Identification
- Antimicrobial Effectiveness Test (AET)
- Antimicrobial Efficiacy
- Assay Development
- Assay Development
- Assay Specificity
- Atomic absorption (AA) spectroscopy
- Bioassay
- Biological Characterisation
- Biophysical
- C-terminal Sequences
- Cell and Molecular Biology
- Cell Characterization
- Cell enzyme bioassays
- Cell Line Development
- Cell proliferation bioassays
- Cell Viability
- Chemical Contaminants
- Chromatography
- Chromatography Purity
- Cleaning Process Validation
- CMC, complement-mediated cytotoxicity bioassays
- Compendial Methods
- contaminant identification
- Crystal Structure Characterization
- Dissolution
- DNA PCR
- Drug Development
- Drug Discovery
- Drug Release/Dissolution
- Dynamic Light Scattering (DLS)
- Electron Microscopy
- Electron Microscopy with Energy-dispersive X-ray spectroscopy (SEM-EDX)
- Elemental Analysis
- Elemental Impurities
- ELISA
- Endotoxins
- ESI-MS
- Experimental Design
- Extractables
- Extractables / Leachables
- Feasibility Studies
- flame atomic absorption spectroscopy (FLAA)
- Flow Cytometry
- forced-degradation stability studies
- Foreign Material Identification
- Formulation Development
- freeze-thaw
- Gas Chromatography
- Gas Chromatography/Flame Ionization Detection (GC/FID)
- Gas chromatography/mass spectrometry (GC/MS)
- Gas Permeability
- GC/MS
- GC/MS (Gas Chromatography/Mass Spectrometry)
- Gel Clot
- Gel Permeation Chromatography
- Gene Expression
- Gene Expression Profiling
- Gene Mutation Assay
- Gene Sequencing
- Glycopeptides
- Host Cell Impurity
- HPLC
- HPLC Gradient
- HPLC-UV
- Immunoassays
- inductively coupled plasma - optical emission spectroscopy (ICP-OES)
- Inductively Coupled Plasma Spectroscopy with Mass Spectrometry (ICP-MS)
- Inorganic Species by ICP-OES
- Ion Chromatography
- Karl Fischer
- Leachables
- Ligand Binding Assays (LBA)
- Liquid Chromatography Tandem Mass Spectrometry (LC/MS/MS)
- Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
- Loss on Drying (under Vacuum)
- Lot Release
- MALDI-TOF
- MALDI-ToF Mass Spectrometry
- Mass Spectrometry
- Method Development
- Method Transfer
- Method Validation
- Microbial Enumeration Test (MET)
- Microbial ID
- Microbial Limit Testing (MLT)
- Minimal Inhibitory Concentration
- Molecular Structure
- Molecular Weight Distribution
- Monograph Verification
- Multi-Angle Light Scattering (MALLS)
- N-terminal Sequences
- N-terminal Sequencing Nano flow RP-HPLC MS/MS Nano spray MS
- Nitrates
- Nitrites
- Nitrosamines
- Non-Routine Analytical
- Non-Volatiles
- Nuclear Magnetic Resonance Spectroscopy NMR
- Odor
- Optical Emission Spectrometry (OES)
- Optical Microscopy
- Optical Rotation
- Osmolality
- Osmolarity
- Package Integrity
- Packaging Analysis
- Particle Analysis
- Particle Identification
- Particle Size Distribution
- Particulate Contamination
- Peptide Mapping
- pH
- Pharmaceuticals
- Photostability
- Physical Chemistry
- Polymerase Chain Reaction PCR
- Polymorphism
- Post Translational Modification Studies (PTM)
- Potency Verification
- Preclinical
- Preservative Challenge
- Process Chemistry
- Process Optimization
- Product Development
- Protein and Peptide Analysis
- Protein Characterization
- Protein Structural Characterization
- Proteomics
- QPCR
- Qualification
- Quality Control
- Rapid Sterility
- Raw Material
- Real-Time Stability
- Refrative Index
- Related Substances
- Research and Development (R&D)
- RNA Analysis
- Robustness
- Scanning Electron Microscopy (SEM)
- Scanning Probe Microscopy (SPM)
- Scanning-Force Microscopy (SFM)
- Seal Integrity
- SEM / Scanning Electron Microscopy
- Semi-Volatiles Analyses
- Simulated Use
- siRNA Interference Assay
- Size Exclusion Chromatography with Multi-angle Light Scattering Detection (SEC-MALS)
- Solid-State Characterization
- Soxhlet Extraction
- Specific Rotation
- Specificity
- Spectroscopy (UV/VIS
- Sterility
- SVOC
- Technology Transfer
- Thermogravimetric analysis (TGA)
- Total Organic Carbons (TOC)
- Trace Elemental Analysis
- Unknowns Identification
- USP Monograph
- UV/Visible Absorption
- Visual Inspection
- Volatile organic compounds (VOCs)
- Wavelength Dispersive X-Ray Fluorescence Spectrometry
- Western Blot
- X-Ray Diffraction
- XRD
- XRF
Publications
Antibody-Drug Conjugates: Overcoming Analytical Challenges in Next-Gen Cancer Therapies (White Paper)Advancing peptide Therapeutics: Challenges and Opportunities in a Booming Market (White Paper)
Understanding and Implementing USP <665> for Single-Use Systems (White Paper)
The Potency Puzzle: Overcoming Challenges in Potency Assays for Cell and Gene Therapy Development (White Paper)
Crystal Clear Vigilance: Detecting Crystallization in ASDs (White Paper)
The high-stakes nature of nitrosamine impurities in APIs (White Paper)
The True Cost of Inadequate E&L Testing (White Paper)
Highly Sensitive Analysis of Glycans in Therapeutic Glycoproteins (White Paper)
Targeted Locus Amplification and NGS combined with qPCR-based breakpoint analysis for the assurance of monoclonality in recombinant cell lines (White Paper)
Solvias to Build Biologics and Cell & Gene TherapyTesting Center in Research Triangle Park (Press Release)
Cergentis is Now Solvias NL (Press Release)
Solvias USA, Inc. Registers with Contract Laboratory - The Laboratory Outsourcing Network! (Press Release)
Solvias to Perform Release Testing on World?s First CRISPR-based Gene-Editing Therapy (Article)
Cergentis is Now Solvias NL (Press Release)
DISCLAIMER: This Laboratory Profile was provided by the company above.
